"Non-catalytic allostery in α-TAT1 by a phospho-switch drives dynamic microtubule acetylation."
Journal of Cell Biology, 2022:221:11:e1-20.
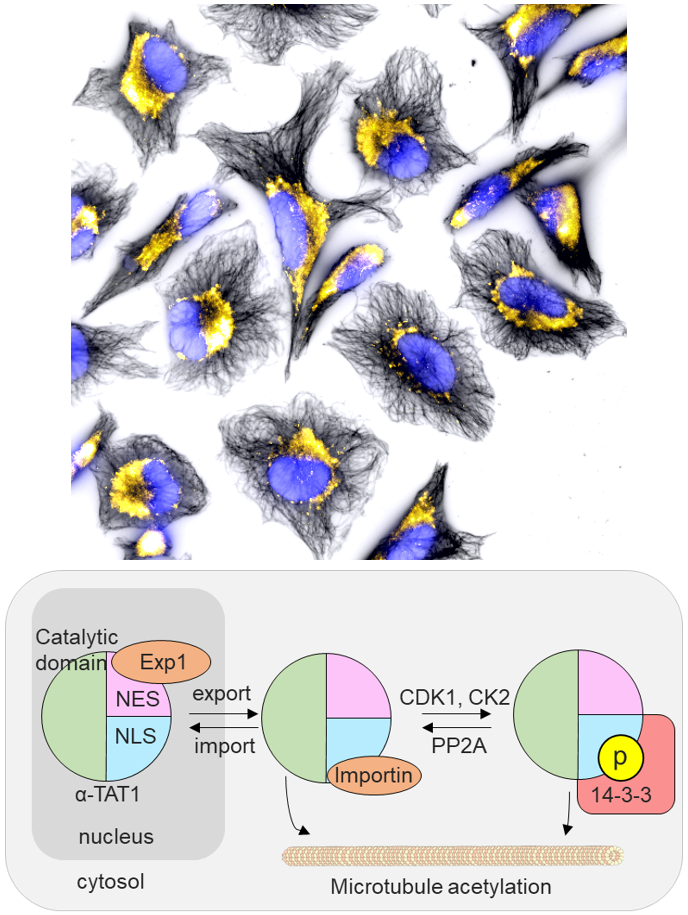
"Location, Location, Location"
We report an unconventional regulatory mechanism of α-TAT1, a posttranslational modification enzyme that acetylates microtubules. More specifically, we identified a unique signal motif in the intrinsically disordered region of α-TAT1. Multidisciplinary characterization subsequently revealed that this previously uncharacterized motif functions as an intricate molecular switch that integrates upstream kinase signaling to actuate the intracellular localization of α-TAT1, hence achieving dynamic microtubule acetylation. Activity of cellular enzymes is often regulated allosterically, where the interaction between a given enzyme and its substrate is initiated by a third molecule binding to the enzyme to induce conformational change. In contrast, it appears to be the spatial regulation that mainly controls the functional output of α-TAT1. While there are enzymes that translocate between nucleus and cytosol, α-TAT1 is the only enzyme to our knowledge that uses the nucleus as a sequestration hideout.
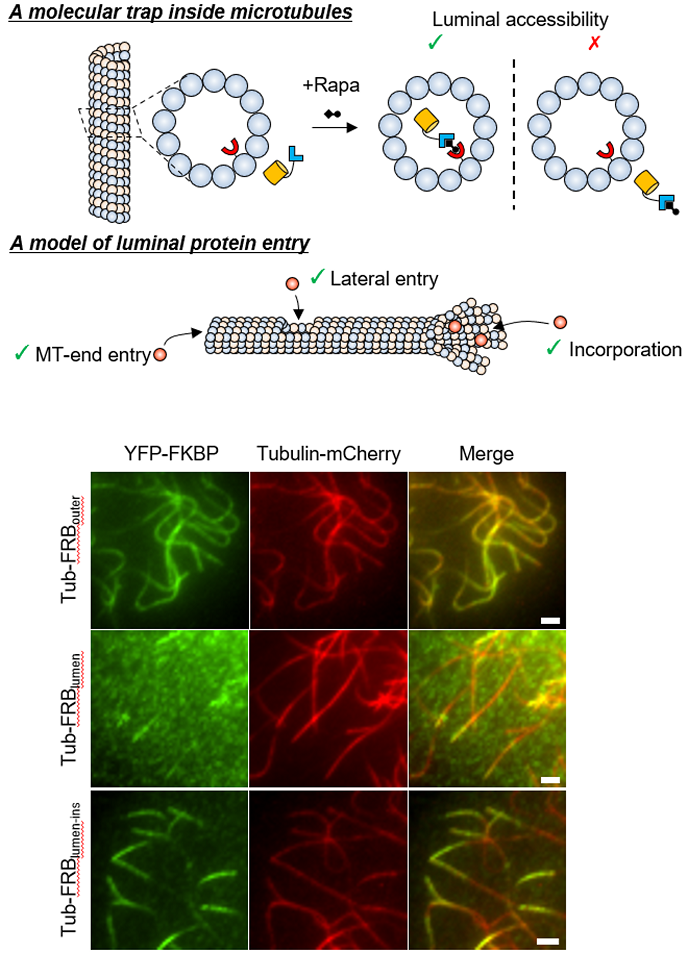
Technological advance: In parallel to characterizing α-TAT1 regulatory mechanisms, we developed a co-recruitment assay based on chemically inducible dimerization tools to assess protein-protein interactions. This assay is like performing Co-IP assays in living cells, and is quantitative, robust and reliable. Most existing protein-protein interaction assays require purification of proteins and/or lysing cells where procedural concerns need to be taken into consideration for data interpretation. Our co-recruitment assays circumvent this problem. And thanks to the modular design principles, the assay is generalizable to virtually any soluble proteins of your choice. Therefore, we expect its wide application to diverse systems. (Photo credit: Abhijit Deb Roy)